Introduction: Myeloproliferative neoplasms (MPNs) are progressive cancers with variable propensity to transform to accelerated phase and blast phase (APBP), an acute leukemia-equivalent state. MPN in APBP is associated with very poor prognosis with shortened survival and is notorious for poor response to therapy. The only curative therapy is allogeneic stem cell transplant.
Blast phase is defined by 20% blasts or higher in the bone marrow biopsy (BMBx) or peripheral blood (PB), while accelerated phase is defined by blast percentage 10% or higher. The diagnosis, and response to therapy often requires serial BMBx and PB testing. There is recognition of excessive circulating PB blasts in MPNs that occasionally exceeds blast count in BMBs, especially in fibrotic bone marrow. The aim of this study is to investigate the correlation between PB and BMBx blasts, and if PB testing might be a more convenient and lest costly marker for diagnosis and response assessment of MPN in APBP.
Methods: This is a cross sectional study, based on single institution retrospective chart review. Subjects included all available subjects over the age of 18, diagnosed with APBP MPN between January 1, 2017, to July 1, 2022. We collected serial blast percentages in simultaneously collected BMBx and PB samples, assessed by the same hematopathologist. We also collected baseline disease characteristics upon transformation to APBP. This is reported descriptively, and statistically analyzed for significance using a paired t-test.
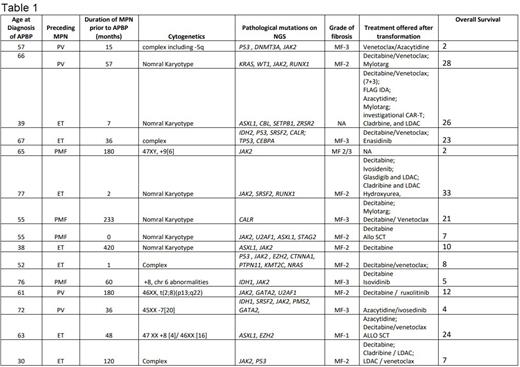
Results: 15 subjects and 54 paired BP and BMBx blast assessments were identified. Median age was 59 (range 30 -71) and median duration since MPN diagnosis was 48 months (1-420). Median OS of the cohort since APBP diagnosis was 10 months. BMBx fibrosis was Grade II-III in 13/15 patients. Cytogenetic and mutation profiles, and therapies delivered are listed on Table 1. Based on evaluation of the results, there was no statistical correlation between blasts in the PB and BMBx both prior to therapy, and during therapy (detailed biostatistical analysis will be provided during ASH presentation)
Conclusions: Patients with APBP MPN undergo frequent diagnostic testing, including a bone marrow biopsy, to evaluate the percentage of blasts in the bone marrow. Quantification of blast count in peripheral blood should not be as a standalone diagnostic lab test for APBP MPN. BMBx remains essential for this diagnosis, response assessment, as well as cytogenetic and molecular profiling. The outcomes on APBP remain poor despite utilization of modern targeted therapeutics.
Disclosures
Yacoub:Apellis: Consultancy; Acceleron Pharma, Inc.: Consultancy; CTI Pharma: Consultancy; Protagonist Therapeutics, Inc.: Consultancy; Servier: Consultancy; Gilead: Consultancy; Novartis: Consultancy; Pfizer: Consultancy; Pharmaessentia: Consultancy; Incyte Corporation: Consultancy; Notable Labs: Consultancy; AbbVie Inc.: Consultancy; AbbVie, Acceleron, Apellis, CTI Pharma, Gilead, Incyte, Notable Labs, Novartis, Pfizer, PharmaEssentia, Servier.: Consultancy.